Atomic Hypothesis
Molecular Nature of Matter
All matter consists of tiny atoms that are always moving. They attract at small separations and repel when squeezed together.
Core idea: constant atomic motion and forces explain the behaviour of all substances.
Gas in Motion
Molecular motion explains why gases fill any shape and flow easily.
Key Ideas
Gas behaviour comes from how its molecules move.
- Molecules are ~10 times farther apart than in solids—almost empty space.
- They move randomly at high speeds in straight lines.
- Elastic collisions with walls create pressure and let gas fill any container shape.
Tip: Higher temperature → faster molecules → greater collision rate and higher pressure.
Ideal Gas Law
Variable Definitions
Applications
Predict Volume Change
Estimate how a gas expands when heated at constant pressure.
Find Moles of Gas
Use measured P, V and T to calculate µ in experiments.
Design Pressurised Tanks
Determine safe storage pressure for a given temperature.
Boyle’s Law Curve
At constant temperature, pressure decreases as volume increases.
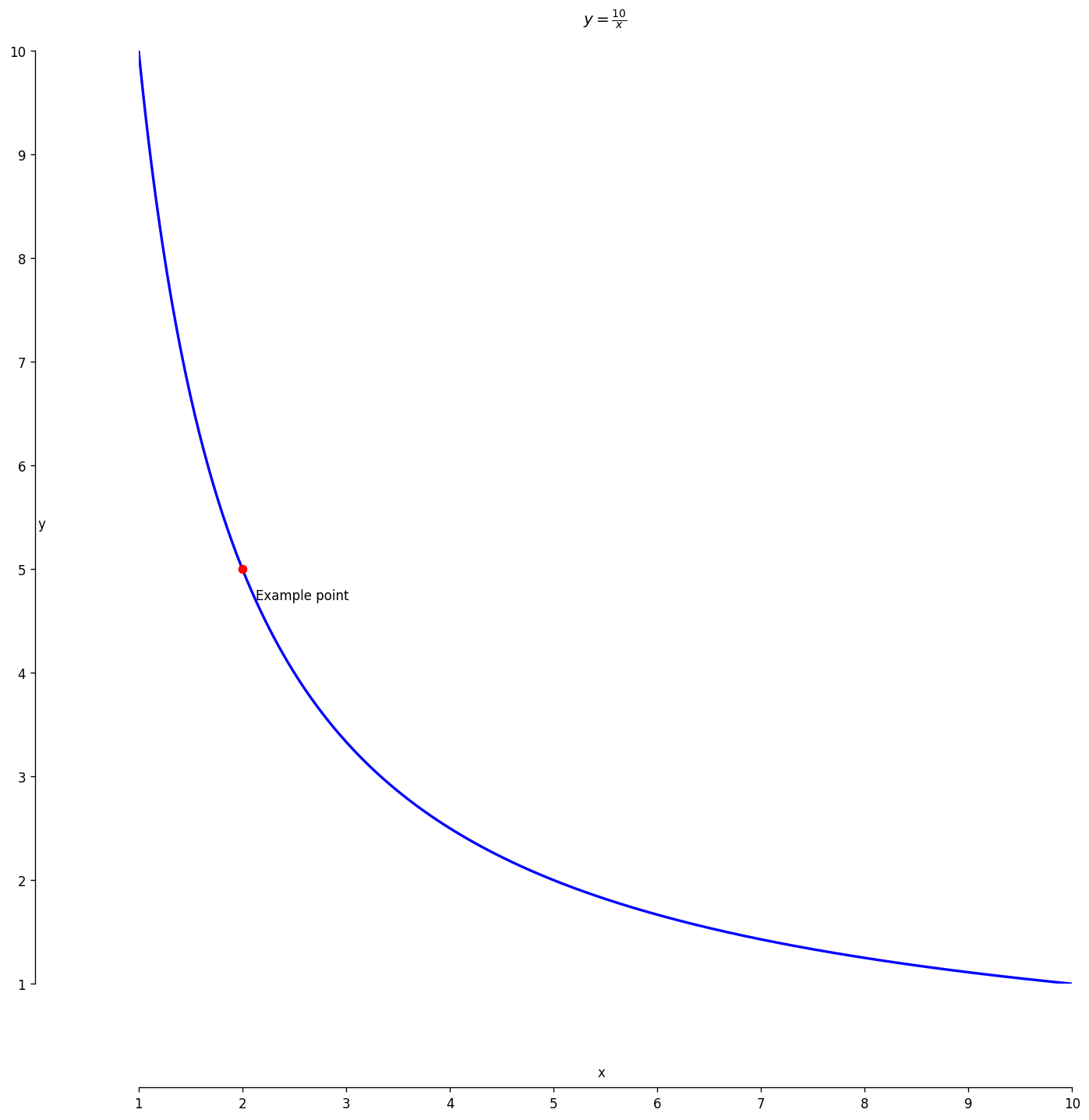
Reading the graph
The curve visualises Boyle’s gas law and its inverse \(P\)-\(V\) link.
- Gas law: \(P \propto \frac{1}{V}\) when temperature is constant.
- Graphical view: a downward-sloping hyperbola.
- Move along the curve: doubling \(V\) halves \(P\).
Tip: The product \(PV\) stays constant for any point on the curve.
Pressure from Motion
We derive \(P = \frac{1}{3} n m v^{2}\) by translating molecular wall hits into measurable pressure.
Momentum kick per hit
A molecule mass \(m\) striking the wall reverses its \(v_x\): change in momentum \(\Delta p = 2 m v_x\).
Hits per second
Number density \(n\) gives \(n A L\) molecules. Half move toward the wall, so hits per second \(=\frac{1}{2} n A v_x\).
Force to pressure
Force = \(\Delta p \times\) hit rate \(= n m v_x^{2} A\). Divide by area and average directions to get \(P = \frac{1}{3} n m v^{2}\).
Check Your Thinking
Question
Which statement best explains why a gas exerts pressure on the walls of its container?
Hint:
Focus on what happens during each molecular collision with the wall.
Correct!
Pressure results from countless collisions that transfer momentum to the container walls.
Incorrect
Remember—gas pressure is due to molecular impacts, not charge, gravity, or volume alone.
Key Takeaways – Recap
Matter is made of discrete atoms or molecules.
Gas particles move randomly and continuously in all directions.
\(PV = nRT\) links pressure, volume and temperature for a fixed amount of gas.
Microscopic motion explains pressure: \(P = \frac{1}{3} n m v^{2}\).
Boyle’s and Charles’ laws confirm kinetic theory experimentally.
Thank You!
We hope you found this lesson informative and engaging.