Matter is Particles
Atomic Hypothesis
All matter is made of tiny particles—atoms or molecules—that move nonstop, attract a little when apart, and repel when crowded.
Think of three everyday objects that must be built from such moving particles.
Ideal Gas Law
This single equation links a gas’s pressure, volume, moles and absolute temperature.
Variable Definitions
Applications
Solve Unknowns
Rearrange to find any missing variable in gas-law problems.
Tyre Pressure vs Heat
Predict how driving warms tyres and raises pressure.
Laboratory to STP
Convert measured volumes to standard temperature and pressure.
Real vs Ideal
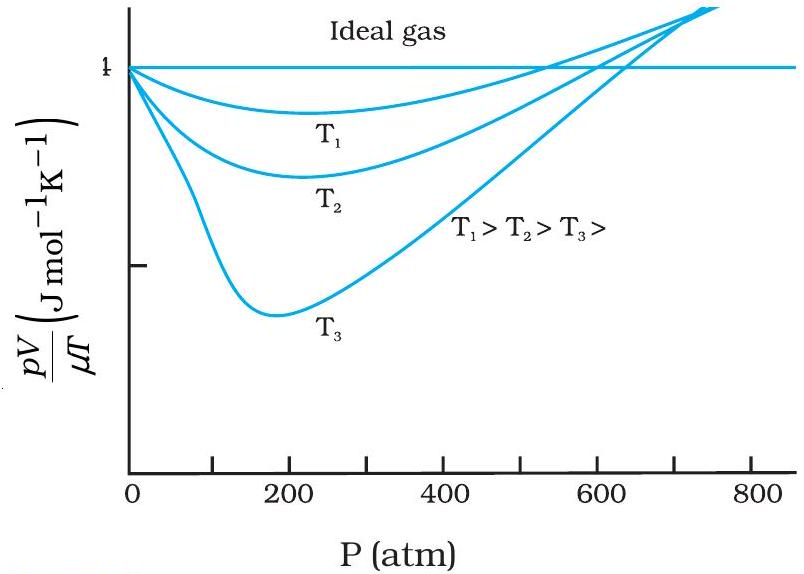
Deviation Curves & Ideal Behaviour
Plot of compressibility factor \(Z\) against pressure shows real-gas deviation curves.
At very low pressure and high temperature, the curve meets the straight ideal line \(Z = 1\), so the gas behaves ideally.
Key Points:
- Deviation curve dips below ideal line: \(Z < 1\) due to attractive forces.
- Curve rejoins at low \(P\) & high \(T\) when forces and collision frequency drop.
- Rise at high \(P\) (\(Z > 1\)) comes from finite molecular volume.
Boyle’s Law
Follow these three quick steps to predict how pressure changes when volume changes at constant temperature.
State the Law
At constant \(T\), gas pressure is inversely proportional to volume: \(P \propto \frac{1}{V}\).
Change the Volume
Halve the volume: \(V \rightarrow \frac{V}{2}\).
Predict the Pressure
Because \(P \propto \frac{1}{V}\), halving \(V\) doubles the pressure: \(P \rightarrow 2P\).
Pro Tip:
Quiz yourself: If the volume doubles instead, pressure drops to half. Why?
Pressure Explained
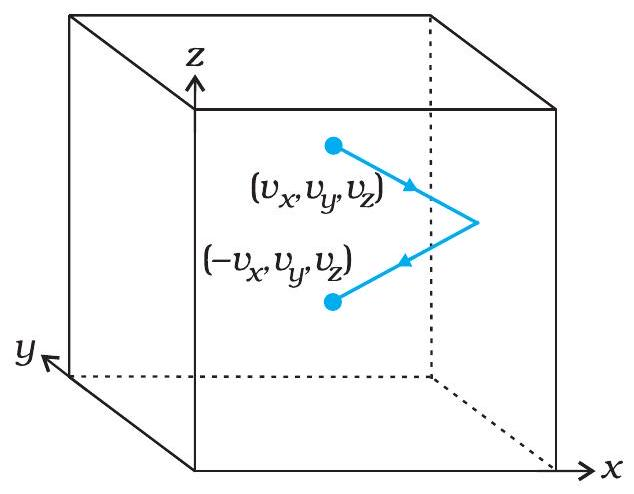
From Collisions to Pressure
Gas molecules move randomly, making countless molecular collisions with the container walls.
Each collision flips the molecule’s perpendicular velocity, delivering a tiny impulse to the wall.
Key Points:
- Momentum change \( \Delta p \) on each hit gives the wall an impulse.
- Many impulses per second create a steady force \( F \).
- Pressure \( P = \frac{F}{A} \) links these wall hits to observable gas pressure.
Temp = Kinetic Energy
Measure Temperature (K)
Record gas temperature in kelvin to link it directly with energy.
Relate KE to T
Use \( \tfrac{1}{2} m v^{2} = \tfrac{3}{2} k_{B} T \); therefore \( KE \propto T \).
Infer Molecular Speed
Higher \( T \) raises \( v^{2} \); molecules move faster in hotter gas.
Multiple Choice Question
Question
If the absolute temperature of an ideal gas triples, what happens to the root-mean-square speed of its molecules?
Hint:
Remember: \(v_{\text{rms}} \propto \sqrt{T}\).
Correct!
\(\sqrt{3}v_{\text{rms}}\) follows because \(v_{\text{rms}} \propto \sqrt{T}\). Good grasp of kinetic energy!
Incorrect
Use \(v_{\text{rms}} \propto \sqrt{T}\); tripling \(T\) multiplies \(v_{\text{rms}}\) by \(\sqrt{3}\).
Key Takeaways
Gas is a collection of randomly moving molecules.
Equation \(PV = \mu RT\) links pressure, volume and absolute temperature.
Low pressure and high temperature give near-ideal behaviour.
Pressure arises from molecules hitting container walls.
Higher temperature means higher average molecular speed.
Thank You!
We hope you found this lesson informative and engaging.