Ideal Gas Model
Ideal Gas
A hypothetical gas whose behaviour is fully described by the kinetic theory.
Core assumptions:
- Molecules are point-like; their own volume is negligible.
- They move randomly in straight lines between collisions.
- Collisions with walls and other molecules are perfectly elastic.
- No intermolecular forces act except during collisions.
Collisions Build Pressure
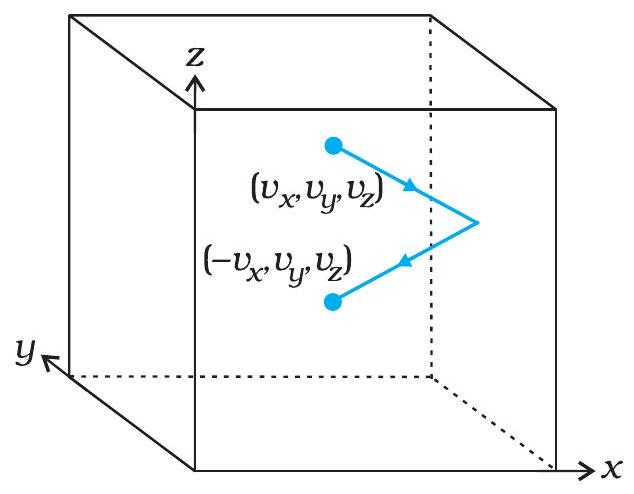
Origin of Gas Pressure
When a molecule strikes the wall, its perpendicular velocity component reverses.
The momentum change \(\Delta p = 2 m v_x\) pushes the wall; countless such pushes per second create measurable pressure.
Key Points:
- Momentum change per collision: \(\Delta p = 2 m v_x\).
- Force equals total momentum transferred to the wall each second.
- Molecular pressure origin: many impacts distributed over the wall area.
Pressure Equation
Microscopically, pressure depends on how many molecules hit the walls and how hard they hit.
Variable Definitions
Applications
Ideal Gas Law Link
Combining with \(PV = NkT\) gives average kinetic energy \( \frac{3}{2}kT \).
Temperature–Pressure Relation
Increasing \( \overline{v^{2}} \) with heat raises pressure at fixed volume.
Root-Mean-Square Speed
Ideal gas law relates macroscopic pressure and volume to particle number and absolute temperature.
Kinetic theory links pressure to the average translational kinetic energy of molecules.
Substitute step 2 into step 1 and cancel \(V\) to relate \(T\) to molecular speed.
Solving shows root-mean-square speed rises with temperature and falls with molecular mass.
Key Insight:
Temperature is a direct measure of the average kinetic energy of gas particles; higher \(T\) means faster molecules.
Real vs Ideal Gas
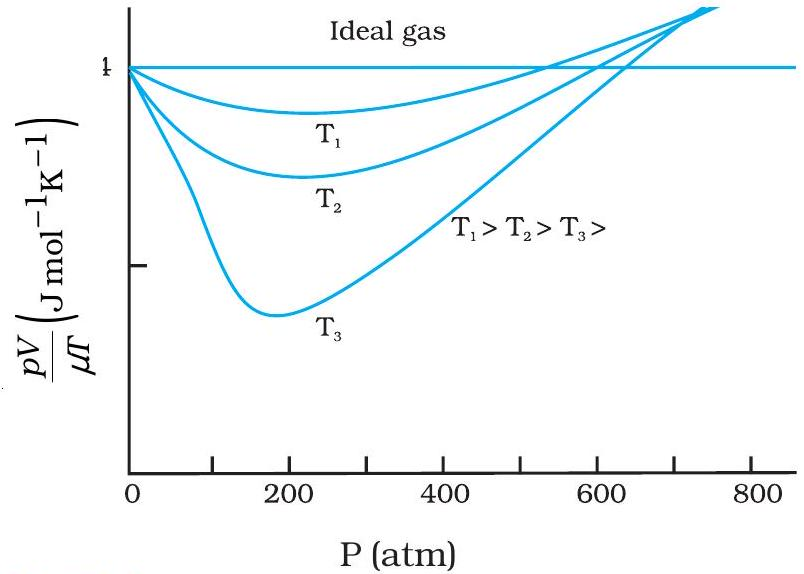
Fig 12.1 Compressibility factor Z vs pressure
Reading Fig 12.1
The flat line at \(Z = 1\) shows an ideal gas.
Real gas curves peel away at high pressure or low temperature because intermolecular forces and molecular volume matter.
Key Points:
- Deviation is negligible at low pressure.
- High temperature reduces attractive forces, giving ideality.
- Use ideal gas law only under these conditions.
Multiple Choice Question
Question
For an ideal gas, if the absolute temperature \(T\) is doubled, what happens to the average translational kinetic energy of its molecules?
Hint:
Use \( \overline{E_k} = \frac{3}{2} k T \). Average kinetic energy is directly proportional to absolute temperature.
Correct!
Since \( \overline{E_k} \propto T \), doubling \(T\) doubles the average kinetic energy.
Incorrect
Remember, average kinetic energy varies linearly with absolute temperature.
Key Takeaways
Molecular Motion (Recap)
Rapid, random molecular collisions with walls create pressure—foundation of microscopic gas view.
Temperature = Energy
Gas temperature directly reflects average translational kinetic energy per molecule.
Micro-Macro Bridge
\(P = \frac{1}{3} n m v^{2}\) mathematically links particle motion to measurable pressure.
Ideal Gas Limit
Point particles with no forces obey \(PV = nRT\), unifying motion and macroscopic law.
Real-Gas Deviations
Departures highlight intermolecular attractions and sizes, sharpening our microscopic understanding.