Atoms Seen!
STM image of a silicon surface; each bright dot is a single atom.
Modern evidence of atoms
Scanning-tunnelling microscopy (STM) resolves individual silicon atoms as bright dots.
Seeing atoms turns a once-theoretical idea into observable fact.
Key Points:
- Quiz: Estimate how many atoms could fit across a human hair.
What is an Atom?
Atom
Smallest particle of an element that keeps the element’s chemical identity in every reaction.
Roughly 10 million atoms lined up equal the width of a human hair.
John Dalton

Portrait of John Dalton (1766–1844)
Father of atomic theory
Early-19th-century chemists needed a model to explain why elements combine in fixed mass ratios.
In 1808, English scientist John Dalton proposed that each element is made of tiny, indivisible atoms unique to it, laying the foundation for modern atomic theory.
Key Points:
- Explained the Law of Constant Proportions using atoms.
- Introduced the Law of Multiple Proportions, linking mass data to atomic ratios.
- First coherent scientific atomic theory—central to modern chemistry.
Dalton’s Postulates
Track how each idea built chemistry’s foundation. By the end, list and critique all five postulates.
Atoms are indivisible spheres
Matter contains tiny, indestructible atoms. Modern science found protons, neutrons, and electrons—atoms are divisible.
Atoms of an element are identical
Dalton assumed equal mass and properties. Isotopes show atoms of one element can differ in mass.
Different elements have different atoms
Distinct atomic masses explain unique element properties. This principle still guides the periodic table.
Compounds form in whole-number ratios
Atoms combine in simple ratios like 2 H : 1 O. Law of definite proportions confirms this statement.
Reactions rearrange atoms, not create them
Mass is conserved during chemical change. Nuclear reactions later revealed exceptions under extreme energy.
Pro Tip:
As you reorder the jumbled list, ask: does modern evidence confirm or revise each claim?
Early Symbols
Dalton’s 1808 table of elemental symbols — hover to see modern letters.
From Sketches to Letters
Dalton used patterned circles to depict the symbolism of elements.
Interpret each pattern by pairing it with today’s one- or two-letter symbol.
Key Points:
- Creative visuals made elements recognisable in small lists.
- System became cumbersome when reactions involved many symbols.
- Modern letters (H, O, Na…) are faster, universal, and easy to type.
Relative Mass Idea
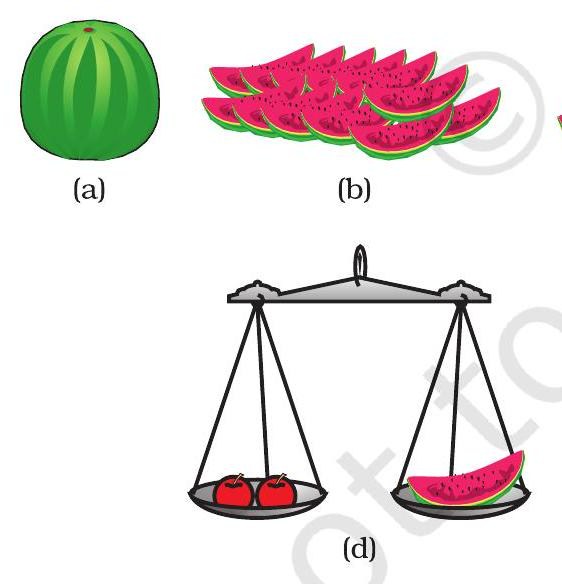
Dalton's balance-scale analogy
Why compare, not weigh?
Atoms are far too light to place on a real balance. Instead, scientists compare one atom's mass with another, just as we compare equal fruit slices.
This comparison gives a relative atomic mass, expressed in atomic mass units (u), making calculations quick and meaningful.
Key Points:
- 1 u is defined as one-twelfth the mass of a carbon-12 atom.
- Relative masses let us rank atoms without knowing their tiny absolute masses.
- Dalton's fruit-slice analogy links laboratory weighing to everyday experience.
Mass Balance Lab
Key Insights
Law of conservation of mass: total mass in a sealed flask never changes.
Move sliders to set volumes; note the initial digital mass.
Press “Mix Solutions”; mass stays constant while a precipitate forms.
Legend
Key Takeaways
Atoms to Mass
Atoms Exist
Experiments prove atoms are real, tiny particles of matter.
Mass Never Disappears
Total mass stays constant in every reaction—Law of Conservation of Mass.
Fixed Ratios
Elements join in constant, whole-number mass ratios—Law of Definite Proportions.
Dalton's Legacy
Dalton unified these laws into atomic theory, guiding modern chemical formulae.